It’s summer, and if you’re like my little boy you enjoy a nice cold glass of homemade lemonade. One day, as he went to add a few drops of lemon, he picked up an oil bottle instead.
Contents
As he started to pour he realized his mistake and stopped immediately. By then it was too late, and a few drops of oil had already fallen into the mixture. He tried mixing it into the water, but the oil drops would not mix.
He scratched his head, looked very confused, and asked me: why do oil and water not mix? After all, he had seen that sugar mixes into the water and that salt also dissolves in water, so why not oil?
I knew this was an excellent opportunity to teach him a bit of science. I thought about it: how do I explain concepts like molecules and structures of atoms to him? I decided that the best way for him to learn was through an experiment.
What Happens When We Mix Water and Oil?
Water and oil do not mix. They are said to be immiscible. This is because of the difference between the chemical structure of water and oil.
Water consists of polar molecules. This means that some of the water molecules have a positive charge and the others have an opposing negative charge. This charge is what binds them together.
Oil, on the other hand, is nonpolar. It does not have positive and negative charged atoms sticking to each other. Therefore, oil can’t break water bonds and mix with water.
This phenomenon causes oil and water to create different visible layers. The denser water molecules sink to the bottom, and the lighter oil forms a layer on top.

Experiment 1: Oil and Water
What You Will Need
- Two transparent glasses
- Water
- Baby oil
- 2-3 different food coloring liquids or paint
- Droppers
The Activity
- Ask your child to take three transparent glasses and add water to them.
- Then add different colors to different glasses with droppers.
- Add baby oil to these glasses and see the magic happen.
- Notice how the oil visibly separates from the colored water.
The Result
My son thought it was some bubbles forming in the water, and he was excited about the result! I explained that these are not regular bubbles that pop up in soda. The oil didn’t mix with the colored water and formed bubbles instead.
What Is An Emulsion?
If you shake the container from the previous experiment, it may appear to mix for a short time. We call this mixture an emulsion. An emulsion is a mixture with two types of liquids mixed in it that should not mix. The moment the mixture is allowed to settle, it again forms two distinct layers.
If you want an emulsion to persist longer, you have to add an emulsifier. This means introducing a molecule that has one polar end and one nonpolar end. Egg yolk and soap are examples of common emulsifiers.
Experiment 2: Making An Emulsion
What You Will Need
- Transparent glass
- ½ cup of oil
- Water
- 1 egg
The Activity
- Ask your child to add the water and egg together in the glass.
- Mixed the oil into it.
- With the addition of egg, the oil and water become thoroughly mixed. There are no separate layers visible.
The Results
With this simple experiment, I was able to explain what an emulsion is.
Like Dissolves Like
We have all heard it: “Like dissolves like.” What does it mean? To understand this, we first need to understand polar and nonpolar molecules.
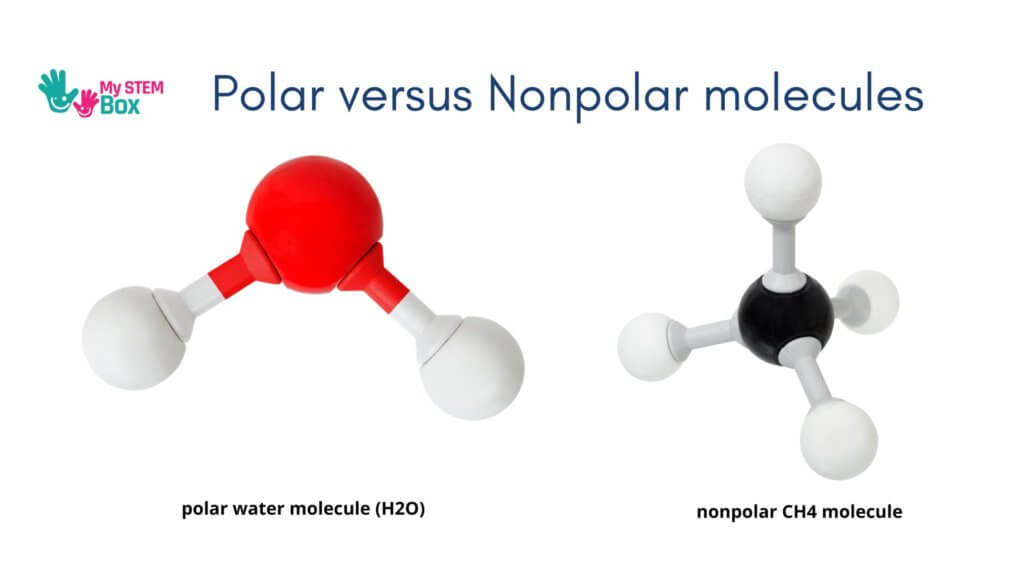
Polar Molecules
A polar molecule consists of oppositely charged “poles.” One pole of the molecule has a positive charge and the other has a negative charge.
Many polar molecules have an asymmetric structure. This means that they have unevenly spaced charges in the molecule. Because of this, the charges won’t cancel out. Some common polar molecules include Water – H2O, Ammonia – NH3, Sulfur dioxide – SO2, Ozone – O3, Ethanol – C2H6O, Sucrose – C12H22O11.
Nonpolar Molecules
Nonpolar molecules either have electrons shared between the atoms or symmetrically spread electrical charges in the molecule, which causes them to cancel out.
Some examples are CH4 – Methane Carbon tetrachloride- CCI4, Benzene- C6H6, noble gasses He, Ne, Ar.
What Mixes With What?
Polar liquids will dissolve other polar molecules. Nonpolar liquids will only mix with other nonpolar molecules. For example, water, which has a polar structure, will mix sugar and coffee, which are also polar. Water will not mix with oil, which is nonpolar.
Why Does This Happen?
Polar substances can form bonds with other polar substances. Their dipolar structure makes it easy for a negatively charged end of one molecule to be attracted to the positively charged end of another molecule and vice versa.
On the other hand, this dipolar attraction works against mixing nonpolar substances with polar ones because they have to compete with the existing dipole-dipole bonds of the polar liquid.
Experiment 3: Like Dissolves Like
What You Will Need
- Two transparent glasses
- Water
- Sugar
- Coffee
The Activity
- Add water to both glasses.
- Mix the coffee into one glass.
- Mix the sugar into the other glass and stir until it dissolves.
The Results
Sugar and coffee both dissolved well with water. No separate layer was visible, unlike what we witnessed earlier with oil. Instead, the water took on some of the white color from sugar and the brown color from coffee: like dissolves like.
Wrap Up
I explained the basic idea of emulsions and polar and nonpolar substances again to my son with the example of how soap dissolves oil and grease.
Soap molecules have two ends, one polar and the other nonpolar. The polar end can dissolve in water, while the nonpolar end can dissolve in oil, breaking it into smaller droplets. This is how soap water can wash away oil and dirt.
I hope these simple experiments help you explain these concepts to your child. Please share your experiences in the comments section below!
As a parent of a five-year-old inquisitive boy, I have gained a lot of experience finding fun activities and toys to help him understand science and understanding our world in general. On this blog, you’ll find an extensive amount of tutorials, guides, and toys about Science, Technology, Engineering, and Math based on my personal experience to help your child develop critical STEM skills.






